Structural insights reveal interplay between LAG-3 homodimerization, ligand binding, and function
in PNAS on March 14, 2024
by Jack L Silberstein, Jasper Du, Kun-Wei Chan, Jessica A Frank, Irimpan I Mathews, Yong Bin Kim, Jia You, Qiao Liu, Elliot A Philips, Phillip Liu, Eric Rao, Daniel Fernandez, Grayson E Rodriguez, Xiang-Peng Kong, Jun Wang, and Jennifer R Cochran
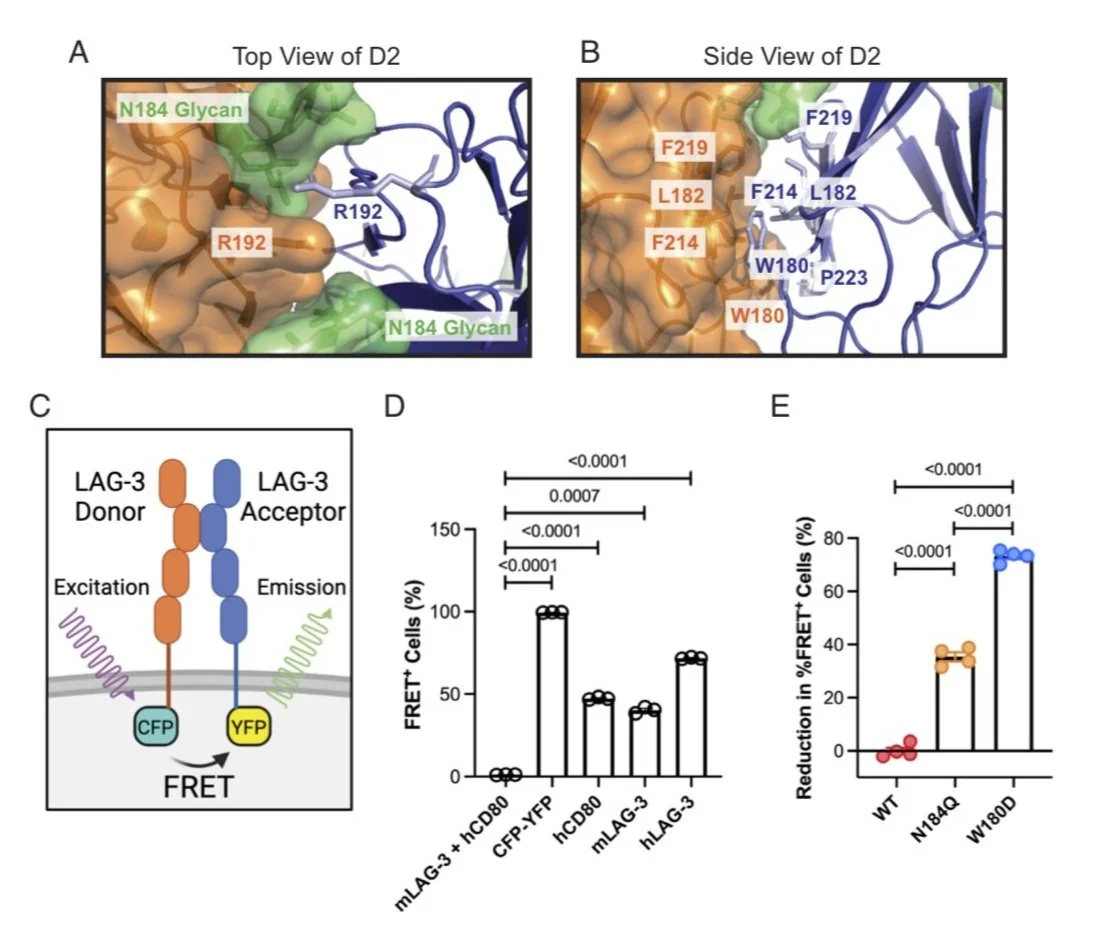
Lymphocyte activation gene-3 (LAG-3) is an inhibitory receptor expressed on activated T cells and an emerging immunotherapy target. Domain 1 (D1) of LAG-3, which has been purported to directly interact with major histocompatibility complex class II (MHCII) and fibrinogen-like protein 1 (FGL1), has been the major focus for the development of therapeutic antibodies that inhibit LAG-3 receptor-ligand interactions and restore T cell function. Here, we present a high-resolution structure of glycosylated mouse LAG-3 ectodomain, identifying that cis-homodimerization, mediated through a network of hydrophobic residues within domain 2 (D2), is critically required for LAG-3 function. Additionally, we found a previously unidentified key protein-glycan interaction in the dimer interface that affects the spatial orientation of the neighboring D1 domain. Mutation of LAG-3 D2 residues reduced dimer formation, dramatically abolished LAG-3 binding to both MHCII and FGL1 ligands, and consequentially inhibited the role of LAG-3 in suppressing T cell responses. Intriguingly, we showed that antibodies directed against D1, D2, and D3 domains are all capable of blocking LAG-3 dimer formation and MHCII and FGL-1 ligand binding, suggesting a potential allosteric model of LAG-3 function tightly regulated by dimerization. Furthermore, our work reveals unique epitopes, in addition to D1, that can be targeted for immunotherapy of cancer and other human diseases.
An engineered interleukin-11 decoy cytokine inhibits receptor signaling and proliferation in lung adenocarcinoma
in Bioengineering & Translational Medicine on July 18, 2023
by Brianna J. McIntosh, Griffin G. Hartmann, Sean A. Yamada-Hunter, Phillip Liu, Camille F. Williams, Julien Sage Jennifer R. Cochran
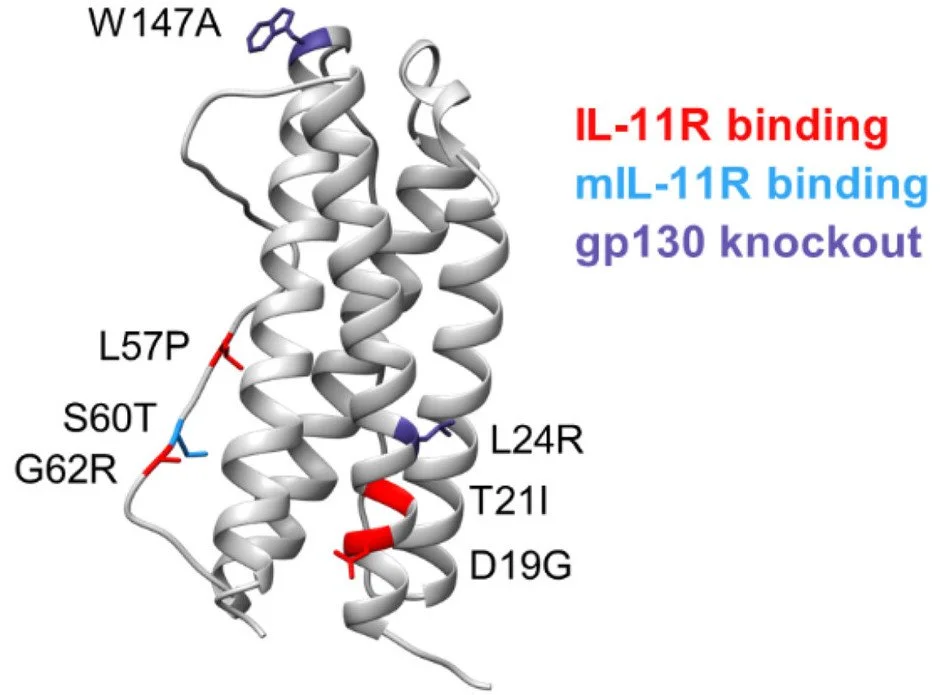
The cytokine interleukin (IL)-11 has been shown to play a role in promoting fibrosis and cancer, including lung adenocarcinoma, garnering interest as an attractive target for therapeutic intervention. We used combinatorial methods to engineer an IL-11 variant that binds with higher affinity to the IL-11 receptor and stimulates enhanced receptor-mediated cell signaling. Introduction of two additional point mutations ablates IL-11 ligand/receptor association with the gp130 coreceptor signaling complex, resulting in a high-affinity receptor antagonist. Unlike wild-type IL-11, this engineered variant potently blocks IL-11-mediated cell signaling and slows tumor growth in a mouse model of lung cancer. Our approach highlights a strategy where native ligands can be engineered and exploited to create potent receptor antagonists.
Targeted TLR9 Agonist Elicits Effective Antitumor Immunity against Spontaneously Arising Breast Tumors
in Journal of Immunology on May 31, 2023
by Caitlyn L Miller, Idit Sagiv-Barfi, Patrick Neuhöfer, Debra K Czerwinski, Carolyn R Bertozzi, Jennifer R Cochran, Ronald Levy
Spontaneous tumors that arise in genetically engineered mice recapitulate the natural tumor microenvironment and tumor-immune coevolution observed in human cancers, providing a more physiologically relevant preclinical model relative to implanted tumors. Similar to many cancer patients, oncogene-driven spontaneous tumors are often resistant to immunotherapy, and thus novel agents that can effectively promote antitumor immunity against these aggressive cancers show considerable promise for clinical translation, and their mechanistic assessment can broaden our understanding of tumor immunology. In this study, we performed extensive immune profiling experiments to investigate how tumor-targeted TLR9 stimulation remodels the microenvironment of spontaneously arising tumors during an effective antitumor immune response. To model the clinical scenario of multiple tumor sites, we used MMTV-PyMT transgenic mice, which spontaneously develop heterogeneous breast tumors throughout their 10 mammary glands. We found that i.v. administration of a tumor-targeting TLR9 agonist, referred to as PIP-CpG, induced a systemic T cell-mediated immune response that not only promoted regression of existing mammary tumors, but also elicited immune memory capable of delaying growth of independent newly arising tumors. Within the tumor microenvironment, PIP-CpG therapy initiated an inflammatory cascade that dramatically amplified chemokine and cytokine production, prompted robust infiltration and expansion of innate and adaptive immune cells, and led to diverse and unexpected changes in immune phenotypes. This study demonstrates that effective systemic treatment of an autochthonous multisite tumor model can be achieved using a tumor-targeted immunostimulant and provides immunological insights that will inform future therapeutic strategies.
Mutational Screens Highlight Glycosylation as a Modulator of Colony-Stimulating Factor 3 Receptor (CSF3R) Activity
in J Bio Chem, 2023
by Michael J. Hollander, Stacy A. Malaker, Nicholas M. Riley, Idalia Perez, Nayla M. Abney, Melissa A. Gray, Julia E. Maxson, Jennifer R. Cochran, Carolyn R. Bertozzi
The colony-stimulating factor 3 receptor (CSF3R) controls the growth of neutrophils, the most abundant type of white blood cell. In healthy neutrophils, signaling is dependent on CSF3R binding to its ligand, CSF3. A single amino acid mutation in CSF3R, T618I, instead allows for constitutive, ligand-independent cell growth and leads to a rare type of cancer called chronic neutrophilic leukemia (CNL). However, the disease mechanism is not well understood. Here, we investigated why this threonine to isoleucine substitution is the predominant mutation in CNL and how it leads to uncontrolled neutrophil growth. Using protein domain mapping, we demonstrated that the single CSF3R domain containing residue 618 is sufficient for ligand-independent activity. We then applied an unbiased mutational screening strategy focused on this domain and found that activating mutations are enriched at sites normally occupied by asparagine, threonine, and serine residues – the three amino acids which are commonly glycosylated. We confirmed glycosylation at multiple CSF3R residues by mass spectrometry, including the presence of GalNAc and Gal-GalNAc glycans at wild-type threonine 618. Using the same approach applied to other cell surface receptors, we identified an activating mutation, S489F, in the interleukin-31 receptor alpha chain (IL-31Rα). Combined, these results suggest a role for glycosylated hotspot residues in regulating receptor signaling, mutation of which can lead to ligand-independent, uncontrolled activity and human disease.